Tablet Hardness, Friability, and Dissolution: Key Quality Parameters in Dietary Supplement Manufacturing
CSK Biotech, a leading tablet supplement manufacturer, ensures top-quality dietary supplements by rigorously testing tablet hardness, friability, and dissolution. These key parameters guarantee product consistency, efficacy, and safety in every batch, supporting your health with trusted quality.
Tablets are one of the most widely used dosage forms in the dietary supplement industry due to their stability, scalability, and ease of handling. To ensure consistent product quality and regulatory compliance, manufacturers monitor several critical physical parameters during production. Among the most important are tablet hardness, friability, and dissolution.
For brand owners and distributors, understanding these parameters helps support informed decision-making when selecting a manufacturing partner such as CSK Biotech.
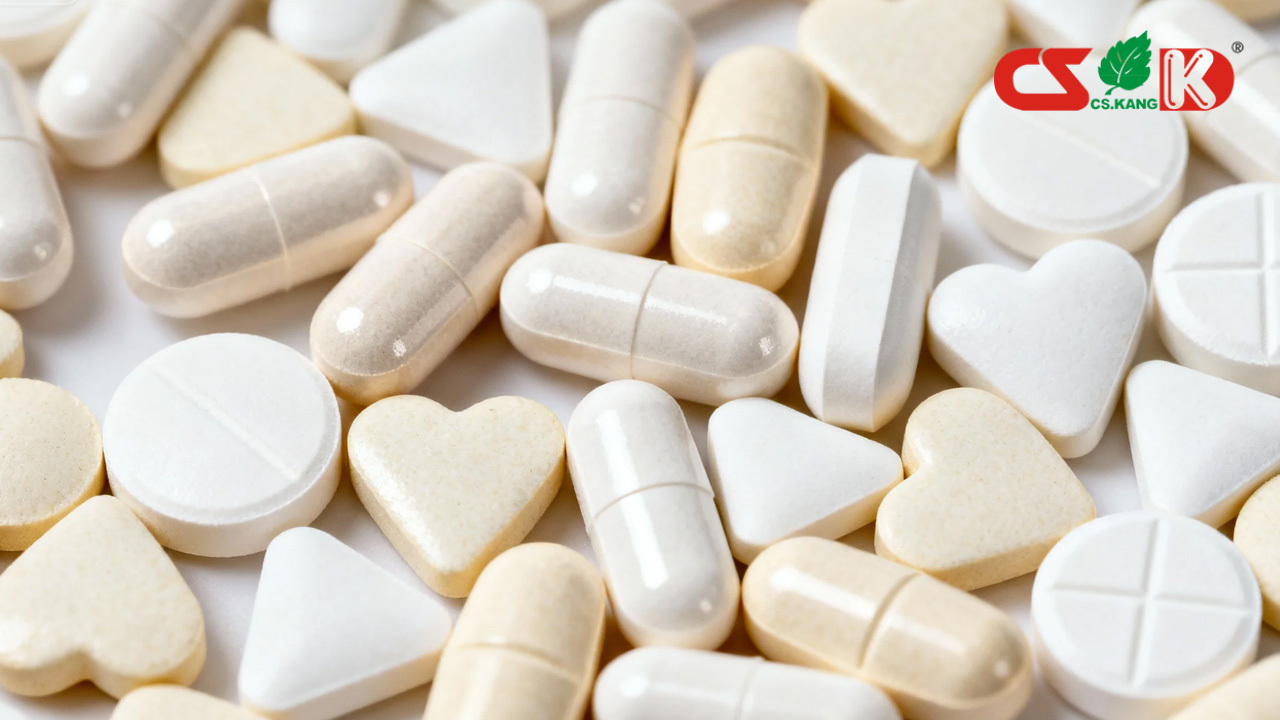
Overview of Tablet Quality Parameters
Tablet quality parameters describe physical and mechanical characteristics that affect manufacturing consistency, packaging performance, and product handling. These parameters are evaluated against predefined internal specifications and relevant pharmacopeial or industry guidelines, where applicable.
They do not imply or guarantee any functional or health-related outcomes.
Tablet Hardness
What Is Tablet Hardness?
Tablet hardness refers to the mechanical strength of a tablet, typically measured as the force required to break it under controlled conditions. Hardness is influenced by formulation composition, compression force, and tooling design.
Why Hardness Matters
Appropriate hardness helps tablets:
-
Maintain structural integrity during handling and packaging
-
Resist damage during transportation
-
Maintain consistent appearance
Excessive hardness or insufficient hardness may affect downstream processing or handling, which is why hardness is carefully monitored during production.
Tablet Friability
What Is Friability?
Friability measures a tablet’s resistance to surface abrasion and chipping during handling. It is usually assessed by rotating tablets in a standardized testing device and measuring weight loss.
Importance of Friability Control
Low friability indicates that tablets can withstand:
-
Bottling and packaging operations
-
Transportation and routine handling
Friability testing supports quality consistency and helps reduce the risk of damaged or visually inconsistent tablets.
Tablet Dissolution
What Is Dissolution?
Dissolution testing evaluates the physical disintegration and dispersion behavior of a tablet under defined laboratory conditions. It is a controlled quality test used to assess batch-to-batch consistency.
In the dietary supplement context, dissolution testing is a quality control tool, not a measure of efficacy or physiological performance.
Factors Influencing Dissolution
Tablet dissolution characteristics may be influenced by:
-
Tablet hardness and compression force
-
Binder and excipient selection
-
Tablet coating type
-
Manufacturing process parameters
Balancing these factors is essential to maintain consistent tablet quality.
Relationship Between Hardness, Friability, and Dissolution
These three parameters are interconnected:
-
Increasing compression force may improve hardness and reduce friability
-
Excessive hardness may alter dissolution behavior
-
Inadequate hardness may lead to higher friability
Manufacturers must optimize formulations and processes to achieve balanced and reproducible results.
Quality Control Practices at CSK Biotech
At CSK Biotech, tablet manufacturing is supported by:
-
GMP-compliant production environments
-
In-process monitoring of compression parameters
-
Defined specifications for hardness and friability
-
Routine quality checks to ensure batch consistency
These practices help support reliable tablet production across a wide range of formulations and formats.
Regulatory Considerations for Global Markets
Tablet quality testing must align with applicable regulatory frameworks, including:
-
US FDA dietary supplement manufacturing requirements
-
EU food supplement regulations
-
Germany’s LFGB standards
-
Relevant Chinese food and health product quality requirements
All testing results and specifications must be documented and presented accurately without misleading statements.
Conclusion
Tablet hardness, friability, and dissolution are essential physical quality parameters in dietary supplement manufacturing. Proper control of these characteristics supports consistent production, efficient packaging, and regulatory compliance across global markets.
By working with an experienced contract manufacturer such as CSK Biotech, brands can develop tablet products that meet defined quality standards while remaining fully compliant with international regulations.
Latest Popular Articles


Discover expert tips from CSK Biotech, one of the top dietary supplements manufacturers, on handling international shipping and compliance for supplements. Ensure smooth global distribution with our comprehensive guide to regulations, packaging, and documentation. Stay compliant and competitive worldwide.
FAQ
文章
Are your manufacturing facilities GMP certified?
Yes. CSK Biotech operates under GMP (Good Manufacturing Practices) standards. Our production facilities follow strict quality · · · management systems to ensure:
-
· Product safety
-
· Batch consistency
-
· Full traceability of raw materials and finished products
How does the cooperation process work?
Our standard cooperation process includes:
-
1.Product consultation and requirement confirmation
-
2.Formula development or review
-
3.Sample production (if required)
-
4.Order confirmation and mass production
-
5.Quality inspection and packaging
-
6.Delivery
This structured process ensures efficiency and transparency.
Why choose CSK Biotech as your manufacturing partner?
Clients choose CSK Biotech because we offer:
-
· Comprehensive dosage form capabilities
-
· Strong customization and R&D support
-
· GMP-compliant manufacturing
-
· Reliable quality and stable supply
-
· One-stop OEM/ODM solutions
Who is CSK Biotech?
CSK Biotech is a professional biotechnology company specializing in OEM/ODM contract manufacturing of dietary supplements. We provide customized production services for softgel capsules, hard capsules, tablets, powders, gummies, and liquid supplements, supporting global brands from formulation to finished products.
Can you help with gummy supplement customization?
Yes. Custom gummy supplements are one of our core strengths. We offer:
-
· Gelatin or pectin-based gummies
-
· Sugar-free or low-sugar options
-
· Customized flavors, colors, shapes, and textures
Our gummies are developed with a focus on taste, stability, and consumer appeal.
What ingredients can be used in your products?
We support a wide range of ingredients, such as:
-
· Vitamins and minerals
-
· Herbal extracts
-
· Amino acids
-
· Probiotics and prebiotics
-
· Functional ingredients for immunity, beauty, sleep, energy, and metabolism
All raw materials are carefully selected and quality-tested before use.
Related Products
Explore our range of complementary dietary supplement products designed to meet diverse formulation and market needs. Each item is developed and manufactured with the same high-quality standards and expertise that define our dietary supplement contract manufacturing services, helping brands expand their product offerings efficiently and reliably.

Request a Quote for Dietary Supplement Contract Manufacturing
If you need CDMO services for health foods, nutritional supplements, or cosmetics, our team of experts is ready to provide you with customized solutions and quotes.



manufactoryinchina
CSK Biotech Dietary Supplements