Stability and Moisture Control in Powder Supplements: Key Manufacturing Considerations
Powder supplements are a widely used format in the dietary supplement industry due to their formulation flexibility and adaptability to various packaging options. However, maintaining stability and moisture control is a critical aspect of powder supplement manufacturing, particularly for products intended for international distribution.
For brand owners and distributors, understanding how stability and moisture are managed helps support consistent product quality and regulatory compliance across global markets.
Understanding Stability in Powder Supplements
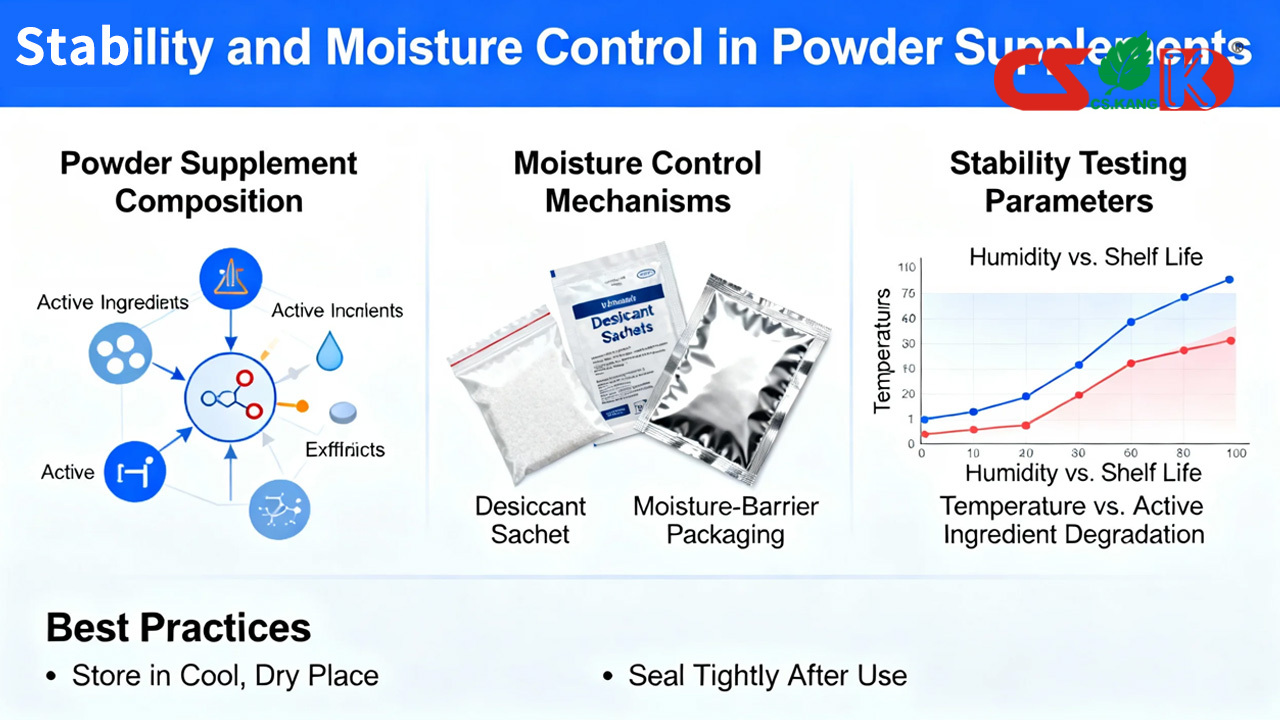
Stability in powder supplements refers to the ability of the product to maintain its defined physical and chemical characteristics within specified limits throughout its intended shelf life, when stored under recommended conditions.
Stability does not imply any functional or physiological effect and is assessed through standardized quality evaluations.
The Role of Moisture in Powder Supplement Quality
Moisture is one of the most influential environmental factors affecting powder supplements. Excess moisture may lead to:
-
Clumping or caking
-
Changes in flowability
-
Variations in appearance
-
Packaging-related challenges
Conversely, overly dry conditions may also affect powder handling properties. Balanced moisture control is therefore essential during both manufacturing and storage.
Common Sources of Moisture Exposure
Powder supplements may be exposed to moisture from multiple sources, including:
-
Hygroscopic raw materials
-
Ambient humidity during processing
-
Packaging materials with insufficient moisture barriers
-
Storage or transportation environments
Identifying and controlling these sources is a key part of quality management.
Manufacturing Controls for Moisture Management
Environmental Controls
Controlled temperature and humidity conditions within production areas help minimize moisture uptake during blending, filling, and packaging processes.
Raw Material Handling
Raw materials are evaluated for moisture sensitivity and stored under defined conditions. Incoming material inspections may include moisture-related specifications to support batch consistency.
Process Design
Manufacturing processes are designed to limit unnecessary exposure to ambient air and to maintain consistent handling times during critical steps.
Packaging Solutions for Moisture Protection
Packaging selection plays a central role in moisture control for powder supplements. Common options include:
-
HDPE containers with moisture-resistant closures
-
Sachets or stick packs with barrier films
-
Use of desiccants where appropriate
Packaging compatibility assessments help ensure that moisture protection aligns with the product’s stability profile and target market requirements.
Stability Testing and Shelf Life Assignment
Stability testing supports the establishment of appropriate shelf life or best-before dates. These studies may include:
-
Real-time stability evaluations
-
Accelerated condition testing
-
Monitoring of physical attributes over time
All stability data must be documented and used in accordance with applicable regulatory frameworks.
Regulatory Considerations for Global Markets
Moisture control and stability management for powder supplements must align with regulations in key regions, including:
-
United States (FDA dietary supplement GMP requirements)
-
European Union food supplement directives
-
Germany’s LFGB standards
-
Relevant Chinese food and health product quality regulations
Labeling related to storage conditions must be factual, clear, and non-misleading.
Stability Management at CSK Biotech
At CSK Biotech, powder supplement manufacturing is supported by:
-
GMP-compliant facilities
-
Controlled production environments
-
Defined quality specifications
-
Packaging and stability planning aligned with international requirements
These measures are designed to support consistent quality and reliable supply for global brand partners.
Conclusion
Stability and moisture control are fundamental aspects of powder supplement manufacturing and distribution. Through appropriate environmental controls, packaging strategies, and documented stability evaluations, brands can better manage product consistency and compliance.
Partnering with an experienced manufacturer such as CSK Biotech helps ensure that powder supplement products are developed and produced with stability, quality, and regulatory alignment in mind.
Latest Popular Articles


Discover expert tips from CSK Biotech, one of the top dietary supplements manufacturers, on handling international shipping and compliance for supplements. Ensure smooth global distribution with our comprehensive guide to regulations, packaging, and documentation. Stay compliant and competitive worldwide.
FAQ
文章
What types of dosage forms does CSK Biotech manufacture?
We offer a full range of supplement dosage forms, including:
-
· Softgel capsules
-
· Hard capsules
-
· Tablets
-
· Powder supplements
-
· Gummy supplements
-
· Liquid supplements (drops, syrups, oral liquids)
This allows our clients to build a complete and diversified product line with one reliable manufacturing partner.
Are your manufacturing facilities GMP certified?
Yes. CSK Biotech operates under GMP (Good Manufacturing Practices) standards. Our production facilities follow strict quality · · · management systems to ensure:
-
· Product safety
-
· Batch consistency
-
· Full traceability of raw materials and finished products
Who is CSK Biotech?
CSK Biotech is a professional biotechnology company specializing in OEM/ODM contract manufacturing of dietary supplements. We provide customized production services for softgel capsules, hard capsules, tablets, powders, gummies, and liquid supplements, supporting global brands from formulation to finished products.
Can you help with gummy supplement customization?
Yes. Custom gummy supplements are one of our core strengths. We offer:
-
· Gelatin or pectin-based gummies
-
· Sugar-free or low-sugar options
-
· Customized flavors, colors, shapes, and textures
Our gummies are developed with a focus on taste, stability, and consumer appeal.
Do you provide packaging services?
Yes. We offer a variety of packaging solutions, including:
-
· Bottles and jars
-
· Sachets and sticks
-
· Bulk packaging
-
· Customized labeling options
Packaging can be tailored to your brand and target market needs.
Does CSK Biotech provide OEM and ODM services?
Yes. CSK Biotech provides both OEM (Original Equipment Manufacturing) and ODM (Original Design Manufacturing) services.
-
OEM: Manufacturing based on your existing formula and specifications
-
ODM: Full product development including formulation, dosage form design, and production
Our R&D team works closely with clients to turn concepts into market-ready products.
Related Products
Explore our range of complementary dietary supplement products designed to meet diverse formulation and market needs. Each item is developed and manufactured with the same high-quality standards and expertise that define our dietary supplement contract manufacturing services, helping brands expand their product offerings efficiently and reliably.




Request a Quote for Dietary Supplement Contract Manufacturing
If you need CDMO services for health foods, nutritional supplements, or cosmetics, our team of experts is ready to provide you with customized solutions and quotes.



manufactoryinchina
CSK Biotech Dietary Supplements